Myocarditis Risk Calculator
Based on real-world data from FDA monitoring systems and published studies. This tool shows your relative risk of myocarditis after mRNA vaccination.
When the first mRNA vaccines rolled out in late 2020, they weren’t just a medical breakthrough-they were a leap into uncharted territory. For decades, vaccines relied on weakened viruses or protein fragments. mRNA changed that. Instead of introducing foreign material, it gave your cells a set of instructions to build a piece of the virus themselves. It worked fast. It worked well. But as millions received these shots and more patients began getting mRNA-based cancer treatments, questions about what happens after the injection started to surface. What are the real side effects? How do we catch the rare ones? And how do we know it’s safe for long-term use?
What You Can Expect Right After an mRNA Shot
Most people experience mild, short-lived reactions. Think of them as your immune system waking up. Pain at the injection site? That’s common-seen in about 77% of people after the first dose of Pfizer’s Comirnaty. Fatigue, headache, muscle aches, and chills follow closely behind. These aren’t signs the vaccine is harmful; they’re signs it’s working. In clinical trials, about 25% of recipients felt tired after the first shot, and that number rose to nearly 28% after the second. Compare that to placebo groups, where only about 15% reported fatigue. The difference is clear. These symptoms usually peak within 24 hours and fade by day three. That’s the pattern for vaccines like Comirnaty and Moderna’s Spikevax. Spikevax, which uses a higher dose (100 μg vs. 30 μg), tends to cause stronger reactions. In early trials, nearly 80% of people reported severe fatigue or fever after the second dose. That’s not normal for most vaccines-but it’s expected for mRNA, because it’s designed to trigger a strong immune response. Unlike traditional vaccines, mRNA doesn’t contain live virus or even the full protein. It’s just a temporary blueprint. That’s why side effects are mostly short-term. Your body reads the message, makes the spike protein, shows it to your immune system, and then breaks down the mRNA within hours. The lipid nanoparticles (LNPs) that carry it dissolve too. There’s no lingering presence in your cells.The Rare but Real Risks
The big concern everyone talks about is myocarditis-heart inflammation. It’s rare, but it’s real. In males aged 12 to 29, the rate is about 40 cases per million second doses of Comirnaty. That’s roughly 1 in 25,000. For women and older adults, the risk is far lower. Most cases are mild. Patients recover quickly with rest and ibuprofen. The CDC reports 98.7% of these cases resolve within 30 days. Still, it’s enough to warrant monitoring. Myocarditis is more common with mRNA than with older vaccine types. The adenovirus-based AstraZeneca vaccine, for example, had a lower rate-just 3.8 cases per million. But it carried a different rare risk: blood clots. mRNA vaccines don’t cause those. That’s a trade-off. Another unusual but documented issue is swollen lymph nodes. Some people notice a lump under their armpit or neck after vaccination. It’s not cancer. It’s just your immune system activating. The CDC says it’s common enough to be listed in vaccine guidance, and radiologists now know to ask about recent vaccination before interpreting scans. Then there’s the menstrual cycle. A 2024 study of over 6 million women found that 3.7% experienced a temporary change in cycle length-usually a delay of a few days. No one lost fertility. No one had long-term issues. It resolved within two cycles. Still, it’s something women noticed. And when thousands share stories online, it becomes part of the conversation-even if science hasn’t yet pinned down a direct cause.How mRNA Is Different From Other Therapies
One of the biggest advantages of mRNA is what it doesn’t do. Unlike gene therapies that insert DNA into your genome, mRNA never enters the nucleus. It stays in the cytoplasm, does its job, and disappears. There’s no risk of altering your DNA. That’s a key safety point. It’s also faster to produce. When the Omicron variant emerged, Moderna made a new vaccine candidate in under 24 hours. The manufacturing process is the same-it’s just the genetic sequence that changes. That’s why companies are now using it for cancer, flu, and even autoimmune diseases. But there are downsides. mRNA is fragile. It breaks down quickly if not kept cold. Comirnaty originally needed -70°C storage. Spikevax only needed -20°C. That’s still colder than most home freezers. Newer formulations are improving this. Some now last weeks at regular fridge temperatures. Delivery matters too. The lipid nanoparticles (LNPs) that carry mRNA into cells are made of four key components: ionizable lipids, phospholipids, cholesterol, and PEG. The ionizable lipids are the most reactive. They’re what cause most of the inflammation. Researchers are now designing new lipids that target specific tissues-like the liver or tumors-instead of flooding the whole body. Early results suggest this could cut side effects by up to 80% in the next five years.
Post-Approval Monitoring: How We Watch for Problems
Approval doesn’t mean the job is done. In fact, that’s when the real monitoring begins. In the U.S., the FDA runs the Sentinel Initiative, which tracks 300 million patient records across hospitals, insurers, and clinics. It looks for spikes in heart issues, neurological events, or unusual hospitalizations after mRNA use. Then there’s v-safe. This is a smartphone-based tool launched by the CDC. Over 6 million people signed up after getting their first mRNA shot. They get daily text check-ins for a week, then weekly ones for months. It’s not perfect-some people drop out-but 87% completed at least seven days of reporting. That’s a gold standard for real-world data. In the U.S., doctors are required to report serious side effects to VAERS within 15 days. As of September 2025, VAERS had received over 1.2 million reports for mRNA vaccines. But here’s the catch: VAERS is open to anyone. A report doesn’t mean the vaccine caused the problem. It just means someone noticed it. That’s why experts use statistical tools like BCPNN to find signals-patterns that appear more often than expected by chance. The biggest gap? Diversity. Pre-approval trials were mostly white, middle-aged adults. Only 9.8% of participants were Hispanic, and just 3.2% were Black. That’s a problem when you’re trying to understand side effects across populations. New trials now require more diverse enrollment, but the data from early use still carries that limitation.What’s Happening Beyond Vaccines
The biggest growth isn’t in vaccines anymore. It’s in cancer. The FDA approved two mRNA cancer vaccines in 2024. One, mRNA-4157/V940 (developed by Moderna and Merck), cut melanoma recurrence by 49% when paired with pembrolizumab. Patients reported mostly mild side effects-fatigue, chills, fever. Only 8.3% had severe reactions, compared to 15.2% with the drug alone. Cancer patients on forums like Smart Patients say the experience is better than chemotherapy. Most describe it as “a bad flu for a day.” A few report nausea or joint pain, but few quit treatment. That’s a big win. Researchers are now testing mRNA for autoimmune diseases like multiple sclerosis and type 1 diabetes. The goal? Train the immune system to stop attacking the body. Early trials are small, but safety looks promising. No cases of cytokine storms. No unexpected organ damage. Just targeted immune modulation.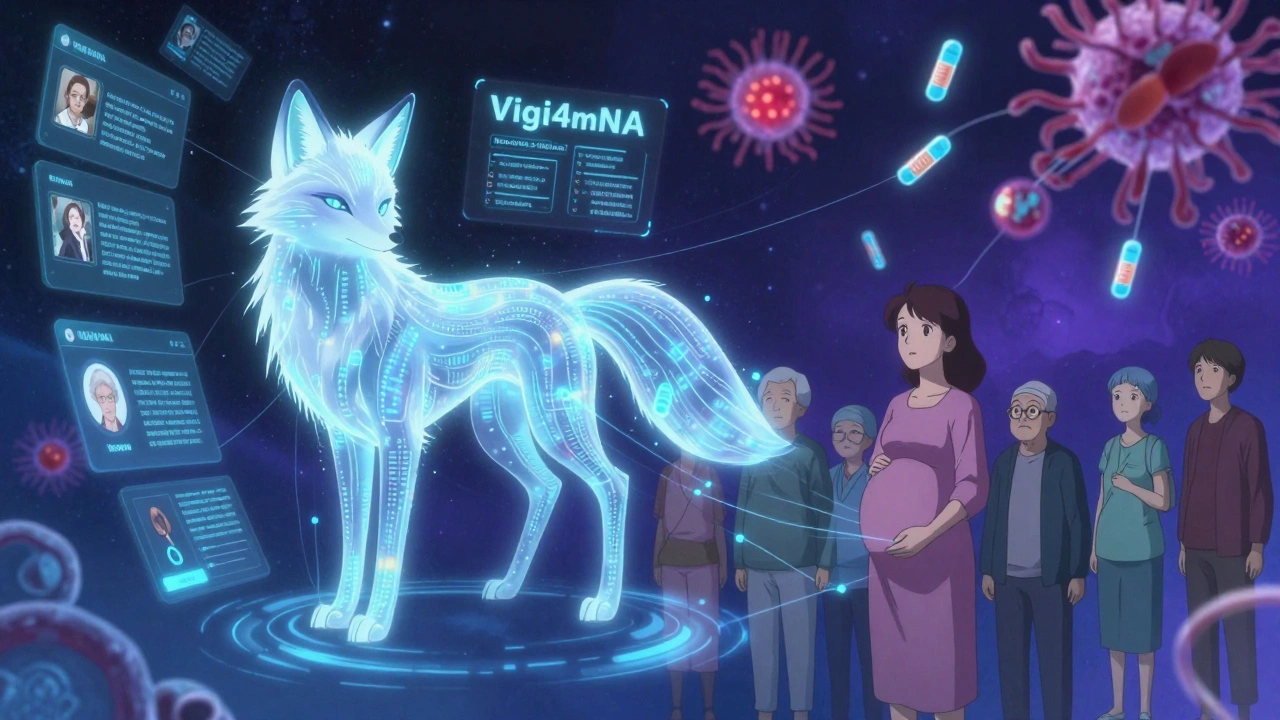
What’s Next: Safer, Smarter mRNA
The next wave is self-amplifying mRNA (saRNA). It needs only 1/10th the dose. That means fewer lipids in the bloodstream. Less inflammation. Fewer side effects. Clinical trials are already underway. AI is helping too. In May 2025, the FDA approved Vigi4mRNA-the first AI system that scans 1.2 million social media posts daily, plus hospital records and pharmacy data, to spot safety signals before they become trends. It flagged a potential link between mRNA and temporary hair thinning in women over 40-something no formal study had noticed yet. Now, researchers are investigating it. The European Medicines Agency launched mRNA-SAFE in January 2025, uniting 27 national agencies to share data and standardize monitoring. That’s huge. No more siloed information. And the future? Tissue-specific delivery. Lipids that only target the liver, or tumors, or the brain. Doses that work at 1-10 μg instead of 30-100 μg. That’s where the real safety improvements will come from.Final Thoughts: Balancing Risk and Reward
mRNA therapeutics aren’t perfect. They cause more short-term side effects than older vaccines. They’re still new. We don’t have 30 years of data. But we have real-world evidence from over 900 million doses given globally in 2024 alone. We have active monitoring systems tracking millions. We have studies showing rare events are manageable and reversible. For cancer patients, mRNA offers hope where none existed. For teenagers, the risk of myocarditis is real-but far lower than the risk of heart damage from a COVID-19 infection. For the elderly, the protection against severe disease outweighs the chance of a few days of fatigue. The goal isn’t to eliminate all side effects. It’s to understand them, manage them, and improve them. And that’s exactly what’s happening.Are mRNA side effects worse than other vaccines?
mRNA vaccines tend to cause more short-term reactions like fatigue, fever, and injection site pain than traditional vaccines like flu shots or inactivated polio vaccines. But they’re similar to viral vector vaccines like Johnson & Johnson’s Janssen. The key difference is that mRNA reactions are more predictable and resolve faster-usually within 48 hours. Serious side effects like myocarditis are rare and far less common than complications from the diseases these vaccines prevent.
Can mRNA change my DNA?
No. mRNA never enters the nucleus of your cells, where your DNA is stored. It works in the cytoplasm, instructing ribosomes to make a specific protein, then breaks down within hours. There’s no mechanism for it to alter your genes. This is a well-established fact in molecular biology and confirmed by dozens of peer-reviewed studies.
Why do some people have swollen lymph nodes after mRNA vaccines?
Swollen lymph nodes are a normal immune response. When your body detects the spike protein made from mRNA, immune cells gather in nearby lymph nodes to respond. This can cause temporary swelling under the arm or neck. It’s not cancer. It’s not infection. It’s just your immune system doing its job. Radiologists now ask patients about recent vaccination before ordering mammograms or CT scans to avoid misdiagnosis.
Is it safe to get mRNA vaccines if I’m pregnant?
Yes. Over 300,000 pregnant women in the U.S. have received mRNA vaccines since 2020. Data from the CDC’s v-safe system and the Vaccine Safety Datalink show no increased risk of miscarriage, preterm birth, or birth defects. In fact, vaccinated pregnant women pass protective antibodies to their babies. The CDC and ACOG strongly recommend mRNA vaccines during pregnancy.
How long does the mRNA stay in my body?
The mRNA itself breaks down within 24 to 72 hours. The lipid nanoparticles that carry it dissolve even faster. The protein your cells make from the mRNA may stick around a little longer-up to a week-but it’s not stored. Once your immune system learns to recognize it, the instructions are no longer needed. That’s why booster shots are needed: the memory lasts, but the mRNA doesn’t.
Are there long-term side effects of mRNA therapeutics?
There’s no biological reason to expect long-term side effects from mRNA. The components don’t linger. The immune response is temporary. Historically, vaccine side effects appear within six weeks. That’s why regulators require six-month follow-ups. For mRNA, the longest data we have is about five years-and no late-onset issues have emerged. Ongoing monitoring continues, especially for chronic use in cancer or autoimmune disease, but so far, the safety profile looks stable.

Victor T. Johnson
December 3, 2025 AT 20:40