What Deep Brain Stimulation Actually Does for Parkinson’s
Deep Brain Stimulation, or DBS, isn’t a cure for Parkinson’s. It doesn’t stop the disease from progressing. But for many people, it changes everything. If you’ve been on levodopa for years and now your movements are a rollercoaster-too stiff one minute, twitching uncontrollably the next-DBS can bring back stability. It works by sending tiny electrical pulses to specific spots in the brain that control movement. These pulses don’t kill brain cells. They don’t remove anything. They simply quiet down the noisy signals causing tremors, rigidity, and freezing.
The two most common targets are the subthalamic nucleus (STN) and the globus pallidus interna (GPi). STN usually lets people cut their medication dose by 30-50%. That means fewer side effects like nausea, hallucinations, or sudden drowsiness. GPi doesn’t reduce meds as much, but it often handles dyskinesias (those involuntary wriggles) better. The choice isn’t just about symptoms-it’s about your life. If you’re already struggling with memory or focus, GPi might be safer. If your main problem is stiffness and slow movement, STN could be the better fit.
Who Is a Real Candidate for DBS?
Not everyone with Parkinson’s qualifies. And too many people wait too long-or never get referred at all. The standard rule? You need at least five years of Parkinson’s and a clear, strong response to levodopa. If you take your medicine and your tremors vanish for hours, that’s a good sign. If you take it and nothing changes? DBS won’t help. The key is this: DBS only works on symptoms that respond to levodopa.
Doctors use the UPDRS-III scale to measure motor improvement. If your score drops by more than 30% after taking levodopa, you’re likely a candidate. But it’s not just about movement. You also need to pass neuropsychological testing. A score below 24 on the MMSE or 21 on the MoCA usually means you’re not a good fit. Why? Because DBS can make memory, attention, or decision-making worse in people who already have mild cognitive issues. If you’re already forgetting where you put your keys or struggling to plan your day, DBS might make it harder.
And then there’s the big one: atypical parkinsonism. If your diagnosis is something like progressive supranuclear palsy (PSP) or multiple system atrophy (MSA), DBS rarely helps. Those conditions don’t respond to levodopa, so they won’t respond to DBS either. Many people get misdiagnosed early on. That’s why a movement disorders specialist-not just any neurologist-is essential before even thinking about surgery.
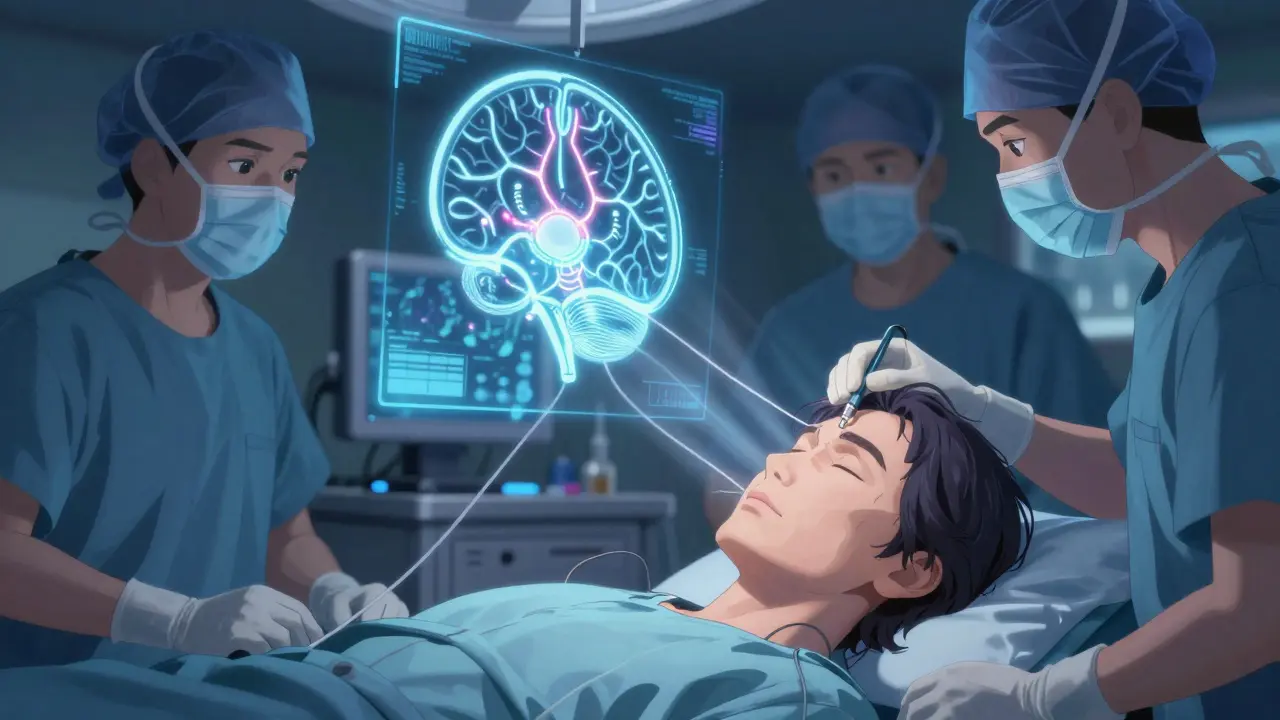
The Surgery: What Happens Inside Your Head
The surgery isn’t done while you’re asleep. You’re awake-partly. That’s because the surgeons need you to move, speak, or blink so they can see exactly where the electrode goes. A metal frame is attached to your head. You lie still while a 3T MRI maps your brain. Then, tiny holes are drilled into your skull. Thin wires, thinner than a pencil lead, are guided to the STN or GPi. Microelectrodes listen to individual brain cells firing. When they hear the right pattern, they know they’ve found the sweet spot.
The whole thing takes 3 to 6 hours. Two sides? That’s two surgeries in one day. Most people go home in 24 to 48 hours. The device itself-the battery pack-is usually placed under the skin near your collarbone or in your abdomen. It’s about the size of a stopwatch. After a few weeks of healing, the device is turned on. But here’s the catch: turning it on is just the beginning.
Programming: The Long Haul After Surgery
DBS isn’t like a light switch. Flip it on, and you’re fixed. It’s more like tuning a radio. You start at one setting. Then, over weeks and months, your neurologist adjusts the voltage, frequency, and pulse width. Sometimes they change which contacts on the electrode are active. Modern leads can steer the current in different directions-like focusing a flashlight instead of using a floodlight. That means fewer side effects and better control.
Most people need 6 to 12 months of regular visits. Monthly at first. Then every few months. Each session can take an hour or more. You’ll need to keep a symptom diary. Note when you’re stiff, when you’re shaking, when your meds kick in. Your doctor uses that to match stimulation to your daily rhythm. If you’re worse in the morning before your pill, maybe the stimulation needs to be stronger then. If your dyskinesia hits after lunch, maybe it’s time to tweak the settings.
And then there’s the battery. Non-rechargeable ones last 3 to 5 years. Rechargeable ones last 9 to 15. But you have to remember to charge them-usually every few days. Miss a week? Your stimulation fades. Some systems now connect to Bluetooth. You can check your settings on your phone. But that doesn’t replace the doctor visits. Programming is still an art, not just a science.
Real People, Real Outcomes
One man in Bristol, 68, had been on levodopa for 12 years. His OFF time was six hours a day. After STN DBS, it dropped to one. His dyskinesias? Gone 90%. He stopped taking three of his five pills. But he started forgetting words mid-sentence. He needed speech therapy. Another woman, 59, got GPi. Her tremors vanished. Her balance didn’t improve much. She still uses a walker. But she could finally dress herself without help.
On online forums, people talk about the highs and lows. One Reddit user said, “My tremors are gone, but planning a meal takes three times longer.” Another wrote, “I thought DBS would stop the disease. It doesn’t. It just makes the good days last longer.” That’s the gap between expectation and reality. DBS doesn’t fix speech, swallowing, balance, or dementia. If those are your biggest problems now, DBS won’t help much.
Studies show 70-80% of well-selected patients get major motor improvement. But 10-15% need hardware revisions-loose wires, infections, broken batteries. And about 30% feel disappointed because their non-motor symptoms didn’t get better. That’s why the team matters. You need a neurologist who knows Parkinson’s inside out. A neurosurgeon who’s done 50+ cases. A neuropsychologist who checks your thinking skills. A coordinator who helps you through the maze of insurance and appointments.
How DBS Compares to Other Options
There are alternatives, but none match DBS for broad motor control. Focused ultrasound (like Exablate Neuro) is non-invasive. But it only treats one side of the body. And it’s mostly for tremor. If you have rigidity or slowness too, it won’t help much. Lesioning surgeries-like thalamotomy or pallidotomy-destroy a small part of the brain. They work, but they’re permanent. If something goes wrong, you can’t undo it. DBS can be turned off. The settings can be changed. The battery can be replaced.
And then there’s the cost. In the U.S., DBS runs $50,000 to $100,000. In the UK, it’s covered by the NHS. But wait times can be long. In the U.S., insurance often requires 3 to 6 months of documented medication trials before approving DBS. Many people give up before they even get to the surgeon.
Medtronic, Boston Scientific, and Abbott dominate the market. Medtronic’s Percept™ PC is the first system that can actually sense brain activity. It detects abnormal beta waves-the same ones that spike when you’re stiff-and adjusts stimulation automatically. It’s called closed-loop DBS. Early results show 27% better symptom control than old-school systems. But it’s new. Not every center has it yet.
Why So Few People Get It
Here’s the sad part: only 1 to 5% of people who qualify for DBS actually get it. Why? Many doctors don’t know the criteria. Some think it’s too risky. Others assume patients are too old. But age isn’t the issue-it’s health. A healthy 75-year-old often does better than a 60-year-old with diabetes and heart disease.
Another reason? Lack of access. Most DBS procedures happen in big academic hospitals. Community neurologists rarely refer. Patients don’t know to ask. The Parkinson’s Foundation calls it “markedly underutilized.” If you’ve had Parkinson’s for five years and your meds aren’t working like they used to, don’t wait. Ask your doctor about a DBS evaluation. It’s not a last resort. It’s a turning point.
What’s Next for DBS
The future is personalized. Researchers are looking at genetics. People with LRRK2 mutations respond better to DBS. Maybe one day, a blood test will tell you if you’re a strong candidate. Digital tools are coming too. Apple Watch can track tremors. Imagine your DBS device adjusting automatically based on your wrist data. Or apps that predict when you’ll go OFF and send a reminder to take your pill or boost stimulation.
There’s also talk of using DBS earlier-before symptoms get severe. The EARLYSTIM-2 trial is testing DBS in people with just three years of Parkinson’s. Early results suggest better long-term outcomes. If proven, it could shift the whole timeline. Instead of waiting until meds fail, you might get DBS when you’re still functional. That could mean years of better quality of life.
But the biggest challenge remains: axial symptoms. Gait freezing, balance loss, swallowing trouble-these don’t improve much with current DBS. That’s where the next generation of targets and tech must focus. Until then, DBS is powerful, but not perfect. It’s not magic. It’s medicine. And when it’s done right, it gives people back control.
Can DBS cure Parkinson’s disease?
No, DBS does not cure Parkinson’s. It doesn’t stop the brain cells from dying or slow the disease’s progression. It only treats the motor symptoms that respond to levodopa-like tremors, stiffness, and dyskinesias. People often expect DBS to fix everything, including memory problems, balance issues, or speech difficulties. But those don’t improve much, if at all. DBS is a tool for symptom control, not a cure.
Is DBS safe for older patients?
Age alone doesn’t disqualify someone. A healthy 75-year-old with good heart and lung function often does better than a younger person with diabetes or high blood pressure. The real risks come from other health conditions, not age. Surgeons assess overall health, not just how old you are. If you can tolerate a 4-hour surgery under local anesthesia, you’re likely a candidate. Many patients over 70 report major improvements in quality of life after DBS.
How long does the battery last in a DBS device?
Non-rechargeable batteries last 3 to 5 years. Rechargeable ones last 9 to 15 years. Rechargeable systems require charging every few days-usually 30 minutes to an hour. If you forget, the stimulation fades. Newer models like Medtronic’s Percept™ PC have longer-lasting batteries and can be monitored remotely via Bluetooth. Battery replacement is a minor outpatient surgery, but it’s still surgery. Planning ahead helps avoid unexpected interruptions in treatment.
What are the biggest risks of DBS surgery?
The most serious risk is bleeding in the brain-about 1-3% chance-which can cause stroke-like symptoms. Infection happens in 3-5% of cases, sometimes requiring hardware removal. Hardware problems like broken wires or misplaced leads occur in 5-15% of patients over time. Some people develop speech problems, balance issues, or mood changes after stimulation. These are often adjustable by reprogramming. The key is choosing a high-volume center: hospitals doing more than 50 DBS procedures a year have 20% fewer complications.
Will I still need to take Parkinson’s medication after DBS?
Yes, but usually much less. Most people reduce their levodopa dose by 30-50%, especially with STN stimulation. This cuts down on medication side effects like nausea, hallucinations, and sudden drowsiness. But you won’t stop completely. DBS doesn’t replace meds-it works with them. Some symptoms, like freezing or balance issues, still need medication. The goal is to find the right balance so you’re stable all day without too many side effects.
How long does it take to see results after DBS is turned on?
You’ll notice some improvement right away-tremors may stop within minutes. But full optimization takes months. The initial settings are just a starting point. Over 6 to 12 months, your team will fine-tune the voltage, frequency, and timing based on your daily symptoms. Many people report the biggest gains after 3 to 6 months. Patience is key. Rushing adjustments can lead to side effects or missed benefits.
Can DBS help with non-motor symptoms like depression or sleep problems?
Sometimes, but not reliably. Some people report better mood or sleep after DBS, likely because their motor symptoms improved and they’re more active. But DBS isn’t designed to treat depression, anxiety, or dementia. In fact, it can sometimes make cognitive issues worse, especially with STN stimulation. If non-motor symptoms are your main concern, DBS is probably not the right choice. Always discuss these with your neuropsychologist before surgery.
What happens if I need an MRI after getting DBS?
You can still get an MRI, but only under strict conditions. Most modern DBS systems (like Medtronic Percept™ PC and Boston Scientific Vercise™) are MRI-conditional. That means you can have a 1.5T or 3T MRI, but only if the device is turned off, the settings are adjusted, and the scan follows specific safety protocols. Never walk into an MRI room assuming it’s safe. Always tell the technician you have a DBS device. Some centers have special protocols. Always check with your DBS team before scheduling any imaging.
Next Steps: What to Do If You’re Considering DBS
If you think you might be a candidate, start with your neurologist. Ask for a referral to a movement disorders specialist. They’ll check your levodopa response and order the right tests. Then, you’ll meet a neuropsychologist for cognitive screening. After that, a multidisciplinary team reviews your case. If you’re approved, you’ll get a 3T MRI and schedule surgery.
Don’t wait until you’re barely able to walk. The earlier you’re evaluated, the better your outcome. And don’t assume your current doctor knows all the options. Many don’t. If you’re not getting answers, go to a larger center. University hospitals with high DBS volumes-like those doing 50+ cases a year-have better results. And if you’re in the UK, NHS referrals can take months. Start early.
DBS won’t fix everything. But for the right person, it’s the difference between being stuck and being free. You don’t need to be perfect. You just need to be a good candidate. And if you are? It might be the best decision you ever make.
Joe Kwon
December 29, 2025 AT 23:52